Botox®
Botox®
Natural Standard Monograph, Copyright © 2013 (www.naturalstandard.com). Commercial distribution prohibited. This monograph is intended for informational purposes only, and should not be interpreted as specific medical advice. You should consult with a qualified healthcare provider before making decisions about therapies and/or health conditions.
Related Terms
BOTOX-A, botulinum toxin type A (BTX-A), Botulinum Toxin Type B (BTX-B), Clostridium botulinum, Dysport.
Background
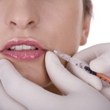
Botox® is a drug that is injected under the surface of the skin to treat several medical disorders related to muscle spasms, muscle tightness and over activity of some glands in the body and is also used cosmetically to treat facial lines and wrinkles. In the United Kingdom and other areas outside of the United States, Botox® is known as Dysport.
The drug Botox® is also known as botulinum toxin, one of the most poisonous naturally occurring chemicals in the world. Botulinum toxin is a protein made by the bacteria Clostridium botulinum. As a drug, botulinum toxin is given in very small amounts, so that nerves around the area of the injection can not receive certain chemical signals.
Clostridium botulinum first appeared in scientific literature in the late 1800s as the bacterial cause of the disease botulism. In 1944, scientists isolated botulinum toxin from Clostridium botulinum. In 1949, the protein was identified as a toxin that blocks messages from the nervous system to the muscles. The first investigational use of the protein in humans under the name botulinum toxin type A (BTX-A) occurred to treat cross eyes in 1989.
In 2002, the U.S. Food and Drug Administration (FDA) approved botulinum toxin type A, now renamed as the drug Botox®, for the treatment of frown lines between the eyes (also called glabellar lines). Since 2002, the drug has also been approved for the treatment of excessive underarm sweating (also called axillary hyperhydrosis), cervical dysplasia (a type of neck spasm), eyelid spasms (also called blepharospasm), and cross eyes (also called strabismus).
Investigational and off-label uses of Botox® include overactive bladder, Parkinson's disease, temporomandibular joint disorder, and excessive salvation. Other cosmetic uses are also under investigation.
Procedure
Botox® is administered under the skin through a very fine needle. Only qualified doctors should perform this procedure. Depending on the medical condition or cosmetic concern, a person may receive one or several injections during an appointment. The medication is injected on the part of the body where the symptoms occur. An appointment usually lasts no longer than one hour. After all of the injections have been given, the patient must stay at the doctor's office for a period of time to ensure that no severe allergic reaction occurs.
Most people experience some bruising and discomfort in the area where the Botox® was injected.
The benefits of a Botox® treatment may last from three to six months. After this period of time, the effect of the treatment wears off. Some people choose to receive another injection of Botox® after this period of time in order to have continued relief from symptoms. Some insurance companies cover Botox® when it is used to treat a medical condition. Treatments that are used cosmetically to improve a patient's appearance are generally not covered by insurance.
Theory/Evidence
Overactive nerve or gland cells: When Botox® is injected underneath the skin, it prevents the nearby cells from releasing a chemical called acetylcholine. When acetylcholine is not released, nerve impulses that might normally cause the patient's gland or muscle cells to behave in a way that causes undesired symptoms, such as excessive sweating or muscle spasms, are blocked. For instance, if Botox® blocks sweat glands from receiving a message to perspire, then sweat glands will not produce sweat. Similarly, if Botox® blocks signals to muscles, then contraction and movement will be prevented, which may lead to a decrease in wrinkling.
Blepharospasm (eyelid spasms): Blepharospasm is condition where the eyes blink rapidly and uncontrollably. These eye movements are caused by twitching in many small muscles in the eyes. This movement causes difficulty in sight, because the eyes are closed much more often than in patients without the condition. As a result, patients may experience difficulty in everyday tasks, such as driving or reading. Botox® may help these patients by blocking the message from the brain that tells the eye muscles to move. When these muscles in the eyes do not move as often, the eyes stay open longer, and often enough for the patient to see with much less difficulty.
Cervical dysplasia (neck spasms): For people with cervical dysplasia (CD), the muscles in and attacheding to the spine in the neck tighten or spasm in unexpected ways. This unexpected movement may feel painful and make it difficult to hold the neck in one position. As a result, the neck muscles may force the head to move in ways that can be awkward and undesired. Keeping the head in one position in order to conduct daily activities such as reading or driving may become difficult. Botox® causes some paralysis in these muscles, so that they can not jerk the head and neck around. The pain caused by unpredictable neck and head movements may decrease, because the muscles causing the sensation are no longer able to move.
Glabellar lines (eye wrinkles): Glabellar lines refer to creases between the eyes. These creases do not cause any medical difficulties, but some people consider them cosmetically unattractive and may wish to have the skin in this area appear smoother. Botox® causes the muscles in this area to relax, and the wrinkles become less noticeable.
Axillary hyperhidrosis (underarm sweating): Some people may experience uncontrollable and excessive sweating under their arms. Although deodorants, antiperspirants and other medications are available, these treatments sometimes do not provide enough relief from the symptoms for some patients. Excessive underarm sweating can stain clothes, cause undesired body odor and may appear unattractive to the patient. Botox® injections in the armpits block the chemical signals that cause the glands to produce more sweat than they should. When the glands in the armpits do not receive this signal, they do not produce sweat, and the patient may experience relief from symptoms.
Strabismus (crossed eyes): Strabismus is a disorder that causes one eye to be constantly or intermittently misaligned compared to the other when focusing. Uneven tightening around some of the muscles that control the eyeballs causes this condition. Botox® is sometimes used along with certain surgeries to weaken the overactive muscles causing the crossed eyes. This may then help the eyeballs return to their normal position.
Restless legs syndrome (RLS): A 2006 observational case series by Rotenberg et al. evaluated the effect of Botox ® on the alleviation of symptoms in three patients with refractory restless legs syndrome (RLS). The three patients in this study experienced relief in the three measures of efficacy: alleviation of symptoms, reduction of medication use and/or reduced daytime sleepiness.
Safety
Some patients may have a severe allergic reaction to Botox. All over body swelling, difficulty swallowing and/or difficulty breathing are the most likely symptoms that an allergic reaction is occurring. Patients who experience any or all of these symptoms after a Botox® injection should have a person nearby call 911 immediately. Patients should not wait to see if the symptoms resolve or try to take themselves to the hospital.
In some cases, Botox® may prevent normally functioning cells that were not causing undesired symptoms (such as muscle spasms, wrinkles or sweating) from receiving chemical messages. As a result, the muscles or the glands other than those causing undesired symptoms may be paralyzed. Therefore, the body part that received the injection may appear abnormal because it cannot function as usual. These effects are said to wear off between six weeks and six months after the Botox® injection was administered.
Patients who are taking medications, drugs, herbs, or supplements containing atropine and/or pralidoxime should not receive Botox® injections. These chemicals increase the toxicity of Clostridium botulinum and may lead to serious and life threatening side effects.
Patients who have been diagnosed with Lambert-Eaton syndrome, myasthenia gravis or Amyotrophic Lateral Sclerosis (ALS, also known as Lou Gehrigs Disease) are at an increased risk of serious and undesired side effects from Botox®.
Author Information
This information has been edited and peer-reviewed by contributors to the Natural Standard Research Collaboration (www.naturalstandard.com).
Bibliography
Natural Standard developed the above evidence-based information based on a thorough systematic review of the available scientific articles. For comprehensive information about alternative and complementary therapies on the professional level, go to www.naturalstandard.com. Selected references are listed below.
American Society of Plastic Surgeons. www.plasticsurgery.org
U.S. National Institutes of Health Clinical Trials Database. clinicaltrials.gov
Food and Drug Administration. www.fda.gov
International Hyperhidrosis Society. www.sweathelp.org
Rotnberg JS, Canard K, Difazio M. Successful treatment of recalcitrant restless legs syndrome with botulinum toxin type-A. J Clin Sleep Med. 2006 Jul 15;2(3):275-8.View Abstract
Copyright © 2013 Natural Standard (www.naturalstandard.com)
The information in this monograph is intended for informational purposes only, and is meant to help users better understand health concerns. Information is based on review of scientific research data, historical practice patterns, and clinical experience. This information should not be interpreted as specific medical advice. Users should consult with a qualified healthcare provider for specific questions regarding therapies, diagnosis and/or health conditions, prior to making therapeutic decisions.
Updated:
March 22, 2017